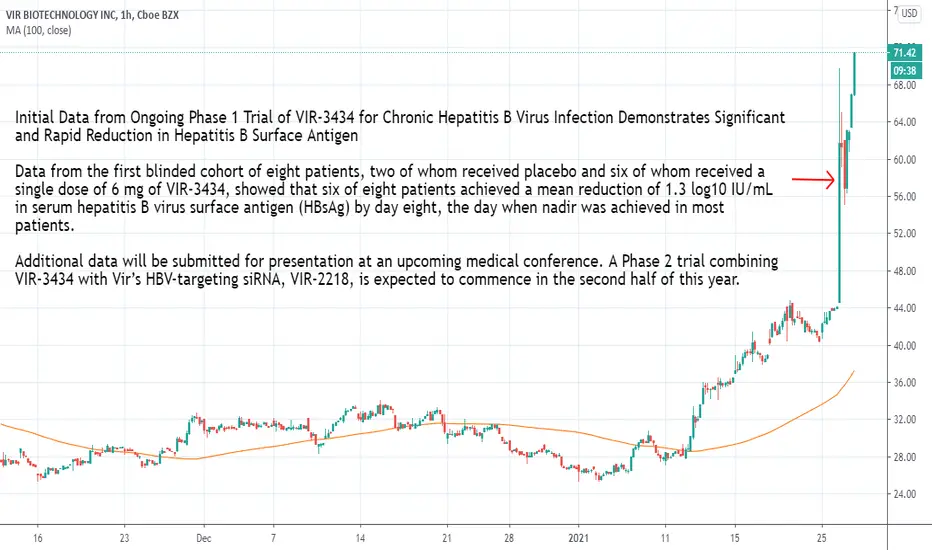
Initial Data from Ongoing Phase 1 Trial of VIR-3434 for Chronic Hepatitis B Virus Infection Demonstrates Significant and Rapid Reduction in Hepatitis B Surface Antigen
Data from the first blinded cohort of eight patients, two of whom received placebo and six of whom received a single dose of 6 mg of VIR-3434, showed that six of eight patients achieved a mean reduction of 1.3 log10 IU/mL in serum hepatitis B virus surface antigen (HBsAg) by day eight, the day when nadir was achieved in most patients.
Additional data will be submitted for presentation at an upcoming medical conference. A Phase 2 trial combining VIR-3434 with Vir’s HBV-targeting siRNA, VIR-2218, is expected to commence in the second half of this year.
finance.yahoo.com/news/initial-data-ongoing-phase-1-130000526.html
Data from the first blinded cohort of eight patients, two of whom received placebo and six of whom received a single dose of 6 mg of VIR-3434, showed that six of eight patients achieved a mean reduction of 1.3 log10 IU/mL in serum hepatitis B virus surface antigen (HBsAg) by day eight, the day when nadir was achieved in most patients.
Additional data will be submitted for presentation at an upcoming medical conference. A Phase 2 trial combining VIR-3434 with Vir’s HBV-targeting siRNA, VIR-2218, is expected to commence in the second half of this year.
finance.yahoo.com/news/initial-data-ongoing-phase-1-130000526.html
Feragatname
Bilgiler ve yayınlar, TradingView tarafından sağlanan veya onaylanan finansal, yatırım, alım satım veya diğer türden tavsiye veya öneriler anlamına gelmez ve teşkil etmez. Kullanım Koşulları bölümünde daha fazlasını okuyun.
Feragatname
Bilgiler ve yayınlar, TradingView tarafından sağlanan veya onaylanan finansal, yatırım, alım satım veya diğer türden tavsiye veya öneriler anlamına gelmez ve teşkil etmez. Kullanım Koşulları bölümünde daha fazlasını okuyun.