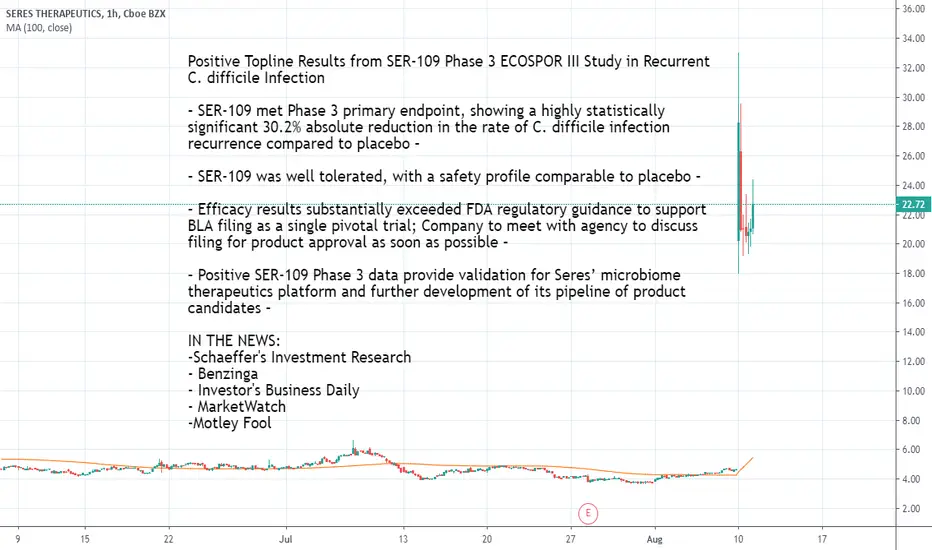
Positive Topline Results from SER-109 Phase 3 ECOSPOR III Study in Recurrent C. difficile Infection
– SER-109 met Phase 3 primary endpoint, showing a highly statistically significant 30.2% absolute reduction in the rate of C. difficile infection recurrence compared to placebo –
– SER-109 was well tolerated, with a safety profile comparable to placebo –
– Efficacy results substantially exceeded FDA regulatory guidance to support BLA filing as a single pivotal trial; Company to meet with agency to discuss filing for product approval as soon as possible –
– Positive SER-109 Phase 3 data provide validation for Seres’ microbiome therapeutics platform and further development of its pipeline of product candidates –
IN THE NEWS:
-Schaeffer's Investment Research
- Benzinga
- Investor's Business Daily
- MarketWatch
-Motley Fool
finance.yahoo.com/news/seres-therapeutics-announces-positive-topline-103000869.html?guce_referrer=aHR0cHM6Ly9maW52aXouY29tL3F1b3RlLmFzaHg_dD1NQ1JCJnR5PWMmcD1kJmI9MQ&guce_referrer_sig=AQAAAE9w_E6Ku0HueBDtCrh4go-VOSqkBrTcmTwKxhzcdOVKPExn5qBDCPxCJSOB3-cXR1Z_IB6IwbBZmL4tu63628Outd7xSvt1h8PWMCk4XopvYnvp_RfZl82mwVfil4rNffHUSoLMmnE6W69qL5tI6yh-UOcevUmfiZBZmMhUqssn&_guc_consent_skip=1597092504
– SER-109 met Phase 3 primary endpoint, showing a highly statistically significant 30.2% absolute reduction in the rate of C. difficile infection recurrence compared to placebo –
– SER-109 was well tolerated, with a safety profile comparable to placebo –
– Efficacy results substantially exceeded FDA regulatory guidance to support BLA filing as a single pivotal trial; Company to meet with agency to discuss filing for product approval as soon as possible –
– Positive SER-109 Phase 3 data provide validation for Seres’ microbiome therapeutics platform and further development of its pipeline of product candidates –
IN THE NEWS:
-Schaeffer's Investment Research
- Benzinga
- Investor's Business Daily
- MarketWatch
-Motley Fool
finance.yahoo.com/news/seres-therapeutics-announces-positive-topline-103000869.html?guce_referrer=aHR0cHM6Ly9maW52aXouY29tL3F1b3RlLmFzaHg_dD1NQ1JCJnR5PWMmcD1kJmI9MQ&guce_referrer_sig=AQAAAE9w_E6Ku0HueBDtCrh4go-VOSqkBrTcmTwKxhzcdOVKPExn5qBDCPxCJSOB3-cXR1Z_IB6IwbBZmL4tu63628Outd7xSvt1h8PWMCk4XopvYnvp_RfZl82mwVfil4rNffHUSoLMmnE6W69qL5tI6yh-UOcevUmfiZBZmMhUqssn&_guc_consent_skip=1597092504
Feragatname
Bilgiler ve yayınlar, TradingView tarafından sağlanan veya onaylanan finansal, yatırım, işlem veya diğer türden tavsiye veya tavsiyeler anlamına gelmez ve teşkil etmez. Kullanım Şartları'nda daha fazlasını okuyun.
Feragatname
Bilgiler ve yayınlar, TradingView tarafından sağlanan veya onaylanan finansal, yatırım, işlem veya diğer türden tavsiye veya tavsiyeler anlamına gelmez ve teşkil etmez. Kullanım Şartları'nda daha fazlasını okuyun.